Studying protein-glycan interactions with 77Se-edited NMR methods
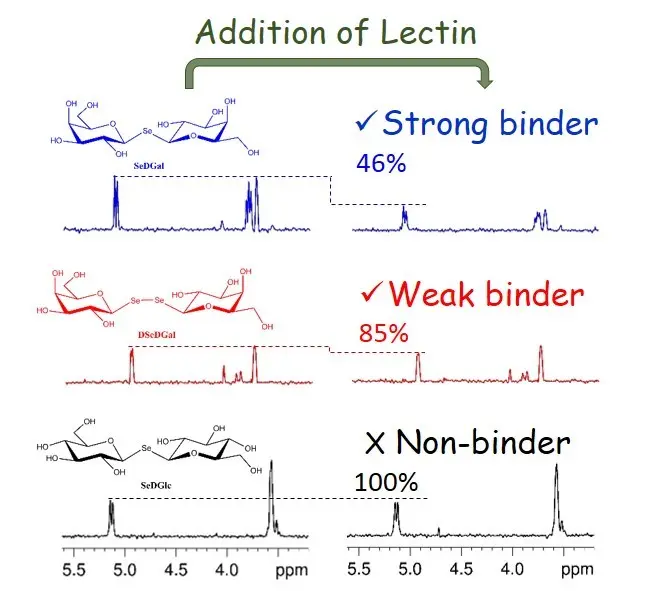 The fundamental importance of protein-glycan recognition calls for specific and sensitive high-resolution methods for detailed analysis. We are working on the development of 77Se NMR-based methods for complementary studies of selenoglycans with superb resolution and optimised sensitivity, where direct NMR detection on 77Se is replaced by indirect observation in a 2D 1H,77Se heteronuclear correlation experiment. The proposed 2D 1H,77Se HSQMBC pulse sequence is based on 1H→77Se CPMG-INEPT out-and-back transfer via 2,3JH,77Se long-range couplings. CPMG-INEPT long-range transfer ensures maximum detection sensitivity, clean signal phases, and reliable ligand ranking. As an example, signal attenuation due to binding induced linebroadening in the 2D 1H,77Se HSQMBC spectrum of a mixture of selenosugars efficiently monitors selenodigalactoside recognition by Galectin-3 protein.
The fundamental importance of protein-glycan recognition calls for specific and sensitive high-resolution methods for detailed analysis. We are working on the development of 77Se NMR-based methods for complementary studies of selenoglycans with superb resolution and optimised sensitivity, where direct NMR detection on 77Se is replaced by indirect observation in a 2D 1H,77Se heteronuclear correlation experiment. The proposed 2D 1H,77Se HSQMBC pulse sequence is based on 1H→77Se CPMG-INEPT out-and-back transfer via 2,3JH,77Se long-range couplings. CPMG-INEPT long-range transfer ensures maximum detection sensitivity, clean signal phases, and reliable ligand ranking. As an example, signal attenuation due to binding induced linebroadening in the 2D 1H,77Se HSQMBC spectrum of a mixture of selenosugars efficiently monitors selenodigalactoside recognition by Galectin-3 protein.
M. Raics, I. Timári, T. Diercks, L. Szilágyi, H.-J. Gabius, K. E. Kövér:
Selenoglycosides as Lectin Ligands: 77Se‐Edited CPMG‐HSQMBC NMR Spectroscopy To Monitor Biomedically Relevant Interactions
ChemBioChem 2019, 20, 1688.
Investigation of heparin-analogue pentasaccharides-antithrombin-III interaction with NMR and molecular dynamic methods
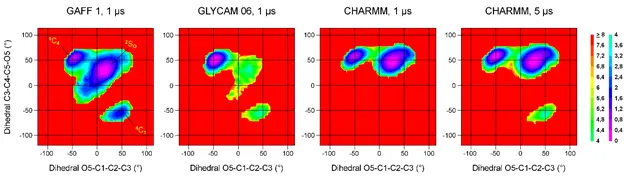
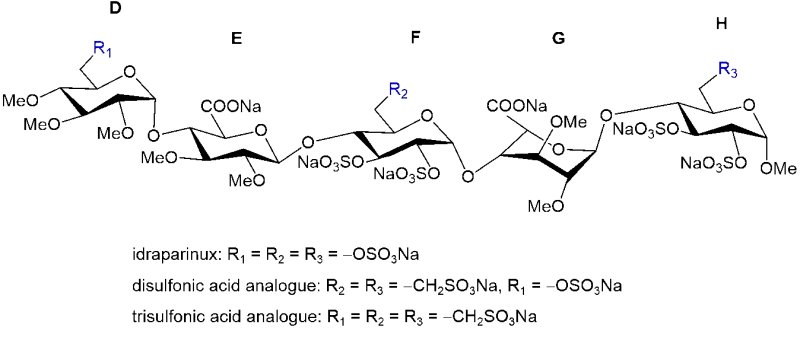 Computational description of conformational and dynamic properties of anticoagulant heparin analogue pentasaccharides is of crucial importance in understanding their biological activities. We designed and synthesized idraparinux derivatives modified with sulfonatomethyl moieties at the D, F and H glucose units that display varied potencies depending on the exact nature of the substitution. We are examining the capability of molecular dynamics (MD) simulations to describe the conformational behaviour of these novel idraparinux derivatives. We used Gaussian Accelerated MD (GAMD) simulations on the parent compound, idraparinux, to choose the most suitable carbohydrate force field for this type of compounds. We compared descriptors obtained from GAMD with NMR spectroscopic parameters related to geometrical descriptors such as scalar couplings and Nuclear Overhauser Effects (NOE) measured on idraparinux. We propose a torsion angle parameter for the sulfonato-methyl group, which was developed for the chosen CHARMM force field using quantum chemical calculations and validated by comparison with NMR data.
Computational description of conformational and dynamic properties of anticoagulant heparin analogue pentasaccharides is of crucial importance in understanding their biological activities. We designed and synthesized idraparinux derivatives modified with sulfonatomethyl moieties at the D, F and H glucose units that display varied potencies depending on the exact nature of the substitution. We are examining the capability of molecular dynamics (MD) simulations to describe the conformational behaviour of these novel idraparinux derivatives. We used Gaussian Accelerated MD (GAMD) simulations on the parent compound, idraparinux, to choose the most suitable carbohydrate force field for this type of compounds. We compared descriptors obtained from GAMD with NMR spectroscopic parameters related to geometrical descriptors such as scalar couplings and Nuclear Overhauser Effects (NOE) measured on idraparinux. We propose a torsion angle parameter for the sulfonato-methyl group, which was developed for the chosen CHARMM force field using quantum chemical calculations and validated by comparison with NMR data.